Is Wine Acidic or Alkaline? [Guide to pH in Winemaking]
Understanding and controlling the pH of wine is a key part of the winemaking process. We explain how to find out the pH of wine and how to change it (and why it matters) in our guide.
Acids appear naturally in grapes but do you need to control their presence in wine? Is the wine you are going to make acidic, alkaline or neutral?
The main three acids found in wines are tartaric, malic and citric.
Before you begin the winemaking process, it's wise to get some more detail on how to get the right balance by the time it comes to drinking.
In our guide, we explain the importance of testing the pH level of wine and what contributes to making wine acidic, alkaline or even basic.
pH and it's Role in Winemaking
One of the most important tests in a winery is measuring the pH of wine (it's level of acidity or alkalinity).
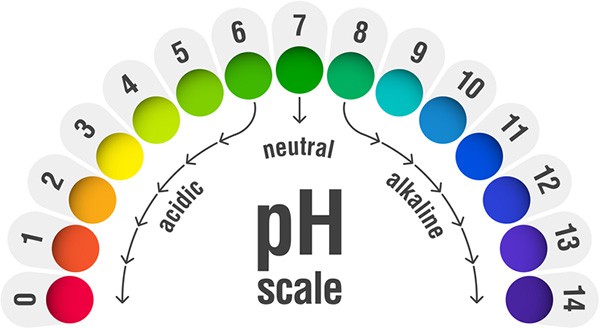
The pH scale goes from 0 to 14. Zero represents an extremely strong acid and at the other end of the scale, 14 is a strong alkali. The middle ground of 7 on the scale is a neutral solution.
In winemaking, the color, oxidation, and chemical (and biological) stability are all influenced by the level of pH.
There are 3 main factors that decide the pH level of a wine:
- The total amount of acid present
- The amount of potassium present
- The ratio of malic acid to tartaric acid
Why is it Important to Control the pH of Wine?
It is important to control the pH level of wines for three reasons:
- Taste
- Microbial stability
- Oxidation
1. Taste
pH affects wine in a couple of ways. A wine with low pH tastes sour or tart. This is because it's too acidic. A lemon is acidic and has a pH of around 2 or 3.
On the other end of the scale, a wine with high pH tastes flat and almost stale.
2. Microbial stability
A wine with higher pH is a haven for microorganisms. This leads to a high risk of spoilage.
Winemakers can keep this under control by adding sulfur dioxide (SO2) to lower the pH, and therefore acting as an antibacterial agent.
3. Oxidation
Wines that have a high pH are more prone to oxidation. Oxidation at this stage is bad as a wine can lose its vibrancy of color and flavor.
This is not the same as aerating a wine just before drinking, which is a good thing.
What's the Ideal pH Range for Wine?
A pH level of 3.2 to 3.6 is the ideal range for good quality wine.
The balancing needs to be accurately as lower than pH3.2 will weaken survival rates of both the malolactic bacteria and the yeast. These two components are an essential part of the fermentation process.
A pH higher than pH3.6 can lead to harmful bacteria flourishing and therefore lead to a high chance of spoilage.
Is pH Alone Accurate Enough?
Wine contains a mixture of acid types, so titratable acidity (TA) is also measured as it shows the amount of tartaric acid present.
Tartaric acid is the dominant acid in wine, but malic, lactic, and citric acids are also present.
Tartaric acid is the dominant acid as it is not consumed in the fermentation process by the yeast. This means it remains in the wine until the end.
The tartaric acid range should be within 7-12 g/l.
The pH scale on its own does not give an accurate measurement of the balance of acids. However, it's a vital measurement for the overall acidity of wine.
Tartaric Acid Explained
Tartaric acid is naturally present in grapes. It's the dominant acid found in wine because it's not consumed in the fermentation process like other acids.
Most other acids are consumed by yeast and microorganisms but not tartaric acid.
This means it's present in the finished product. It can sometimes actually be visible in cold wine in the form of acid crystals. These are roughly the size of sugar grains.
These can be removed in a process called cold stabilization but they aren't going to do you any harm.
How can the TA and pH levels be Altered?
Thankfully, both the pH and tartaric acid levels can be adjusted in a very convenient way.
If you need to increase the acidity in red wines, you can add tartaric powder.
For white wines, a mixture of one-third malic acid to two thirds tartaric acid should work well.
However, it's not easy to forecast how a tartaric acid addition will adjust the pH. Even some expert winemakers struggle to get it right, and in the end, it comes down to your past experience.
It's best to get wine measured for acidity in titratable acidity (g/l), as it will give you more accurate results.
You can get the tests done in good wine laboratories, where they will do an analysis for you.
As soon as you know the amount of TA present in the wine, you will have an idea of how much acid needs to be added to adjust the tartaric acid per liter of wine
A rough range of TA and pH for reds and whites in general is:
Conversely, if you need to reduce the pH and make a wine more acidic there are a few options too:
- Add calcium carbonate (chalk) - this is not the first choice for wineries as it takes a long time to take effect. It must be done at least 3 months before bottling or the chalk may cause a cloudiness in the wine.
- Cold stabilisation - this should be done immediately after fermentation. The wine is exposed to temperatures as close to freezing as possible for at least 2 weeks. Learn more about it here.
- Add Potassium Carbonate (KCO3) - this works like calcium carbonate but reacts immediately and with no deposit.
How to Measure pH in Wine
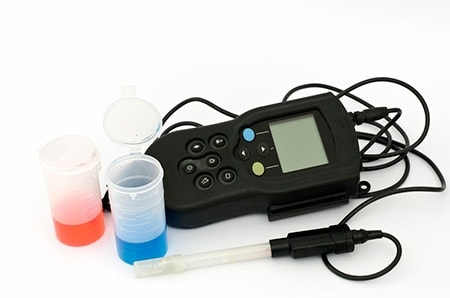
A pH meter is the best way to accurately measure the acidity level of wine during the winemaking process.
There are 2 types of electronic pH meter available. These are much more accurate than cheaper pH strips.
Digital pH Meter: An electronic hand-held pH meter. These meters are highly accurate with great resolutions.
Automatic pH Meter: These allow the automatic pH standardization at pH3 and pH7. The meters are smart enough to tell the users if they detect any dirty electrodes so you can rest assured that you will get the most accurate reading almost every time.